- 4DMedical’s (4DX) XV LVAS offering can now be commercialised in Australia following approval from the Therapeutic Goods Administration (TGA)
- The technology converts X-ray images into four-dimensional data which allows physicians to better diagnose and treat patients with respiratory diseases
- The TGA approval follows an FDA 510(k) clearance back in May which supports the global rollout of 4D’s flagship technology
- 4DMedical is now establishing a sales and marketing team to deliver customers in 2021
- Company shares are trading 12.8 per cent higher for $1.72
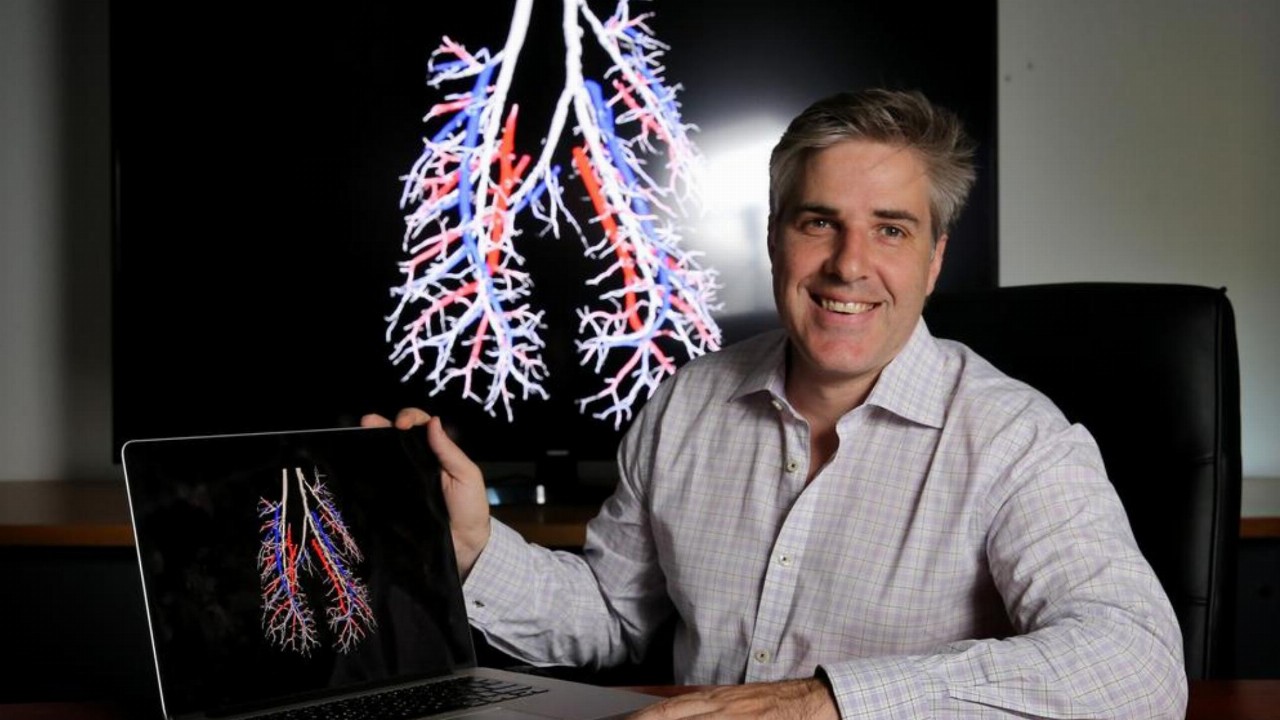
4DMedical’s (4DX) XV LVAS offering has received approval from the Therapeutic Goods Administration (TGA) for commercial use in Australia.
XV LVAS, or XV Lung Ventilation Analysis Software, utilises 4D’s XV Technology platform and converts sequences of X-ray images into four-dimensional data which allows physicians to better diagnose and treat patients who have respiratory diseases.
Now that its XV LVAS software-as-a-solution (SaaS) offering has been given TGA approval, 4DMedical plans to define a path for health departments, labs, medical practitioners and health care professionals to adopt its product.
“We are extremely pleased to announce TGA approval for our XV LVAS offering ahead of schedule, allowing us to offer our world-class technology to our own backyard in Australia,” Founder and CEO Andreas Fouras said.
The TGA approval follows the company receiving an FDA 510(k) clearance back in May which supports the global rollout of 4D’s flagship technology.
The global respiratory diagnostic market is worth an estimated US$31 billion (roughly A$43.4 billion) per annum. The market is dominated by three procedures which are pulmonary function tests, X-rays and computed tomography (CT).
4DMedical believes its technology has compelling market advantages including a non-invasive modality for physicians to understand lung motion and airflow and to identify deficiencies early on. It is also designed to be fully compatible with existing hospital and clinical equipment which means no further capital expenditure is needed.
“Whilst the US market will continue to be our focus as the largest respiratory market, Australia is our home and we believe we can now move quickly to get traction through leveraging our hometown advantage and relationships faster than any other market.
The company is now forming a sales and marketing team to deliver customers in 2021.
Company shares are trading 12.8 per cent higher for $1.72 at 11:23 am AEST.