- Recce Pharmaceuticals’s (RCE) Recce 327 antibiotic is showing promise in treating gonorrhoea, a bacteria which is increasingly antibiotic-resistant
- Already, the bacteria has developed resistance to all but one class of antibiotics, making the search for a new treatment a global priority
- A trial in mice tested different doses of the compound Recce 327 and compared it to Meropenem, a market approved drug
- As the dose of Recce 327 increased the bacterial count was significantly reduced and even more so than in mice treated with Meropenem
- Recce Pharmaceuticals closed 5.3 per cent higher at 39.5 cents each
Recce Pharmaceuticals’s (RCE) Recce 327 antibiotic is showing promise in treating gonorrhoea, a bacteria which is increasingly antibiotic-resistant.
The bacteria neisseria gonorrhoeae has developed resistance to all but one class of antibiotics and has been listed as a significant threat to human health by the World Health Organisation.
“There is an urgent need to find a new class of effective antibiotics to kill the pathogen before it develops resistance to the last recommended treatment,” said Recce Pharmaceuticals Non-Executive Chairman Dr John Prendergast.
“Data from this study, along with previous other reports, continue to highlight the potential of RECCE 327 to not only become a potent broad-spectrum antibiotic but most critically to continue working against antibiotic-resistant bacteria or superbugs, even with numerous repeated uses,” he added.
The study in mice was conducted by an independent Contract Research Organisation and was designed to test the dose-dependency of Recce 327 against neisseria gonorrhoeae.
In the trial, mice were given either a market-approved drug called Meropenem or different doses of Recce 327 twice daily for seven days, starting two days after the initial infection.
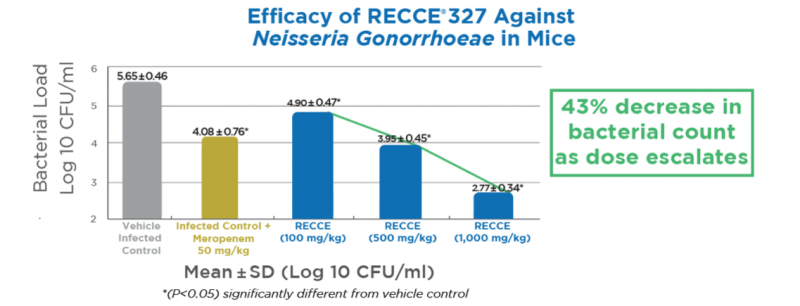
The results showed that the extent to which Recce 327 reduced bacteria was dependent on the dose, as the study director explained.
“RECCE 327 showed a significant dose-dependent antibacterial effect in vaginal load at 100, 500 and 1000 mg/kg given by intravenous bolus when compared to the control group seven days post-infection.”
As the dose increased the bacterial count was significantly reduced and with the higher doses, even more so than in mice treated with Meropenem.
Recce Pharmaceuticals closed 5.3 per cent higher at 39.5 cents each.







