Thr primary endpoint met in Paradigm’s phase 2a randomised placebo-controlled clinical trial of patients with Ross River VirusiPPS was injected to reduce Ross River symptoms- After 12 week follow-up showed 73 per cet of patients showed near remission after taking iPPS
Paradigm Biopharmaceuticals has reported its met the primary safety endpoint in pilot phase 2a randomised; a double-blinded clinical trial for patients with Ross River
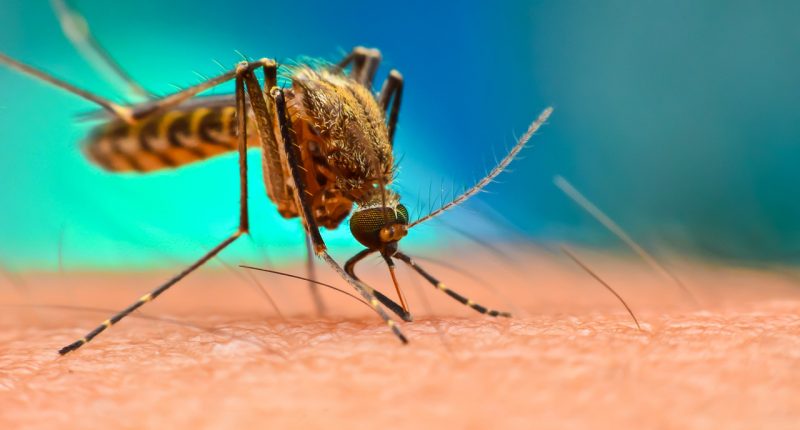
Ross River virus is a mosquito transmitted virus that causes epidemic poly-arthritis and arthralgia with around half of the patients experiencing a fever and rash.
Joint pain is present in more than 95 per cent of cases and is mostly experienced in the fingers, toes, ankles, wrists, elbows and knees. So far no treatments have been known to shorten the duration however some aspirins provide limited relief.
Participants with Ross River induced joint stiffness were treated with injectable pentosan polysulfate sodium (iPPS). These participants met the aims for the pilot study by demonstrating safety outcomes and reduced disease symptoms.
PPS is a semi-synthetic drug manufactured from European beech xylan which produces a negatively charged product that mimics glycosaminoglycan, repeating sugar units.
Currently, PPS is not registered in Australia but is registered in four of the seven major global pharmaceutical markets. Paradigm is also using PPS to treat patients with knee osteoarthritis.
A total of 18 patients took part in the trial, 11 iPPS treated and seven placebo, and the aim of these tests was to demonstrate the safety of iPPS in patients with chronic and sustained Ross River symptoms.
A total of 90 per cent of patients in the iPPS group, 10/11, and 100 per cent of patients in the placebo group were categorised with clinical disease severity ranging from low, moderate and high severity.
After a 12 week period 73 per cent, 8 out off 11, patients in the
These positive results have confirmed pre-clinical findings of the safety and efficiency of iPPS and Paradigm will now enter discussions with the United States Department of Defence.