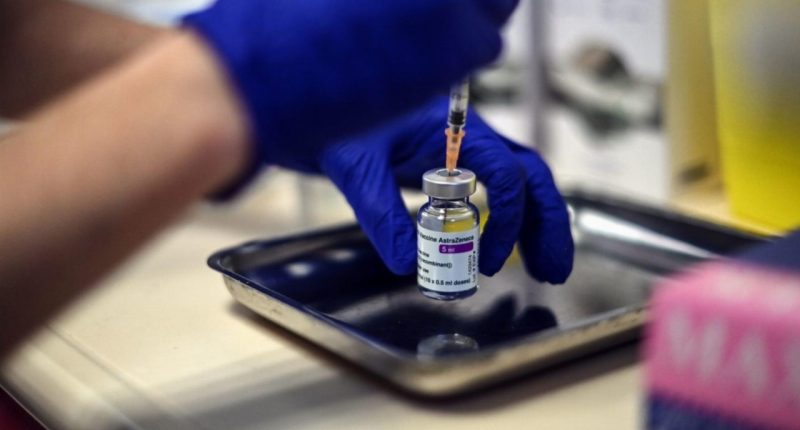
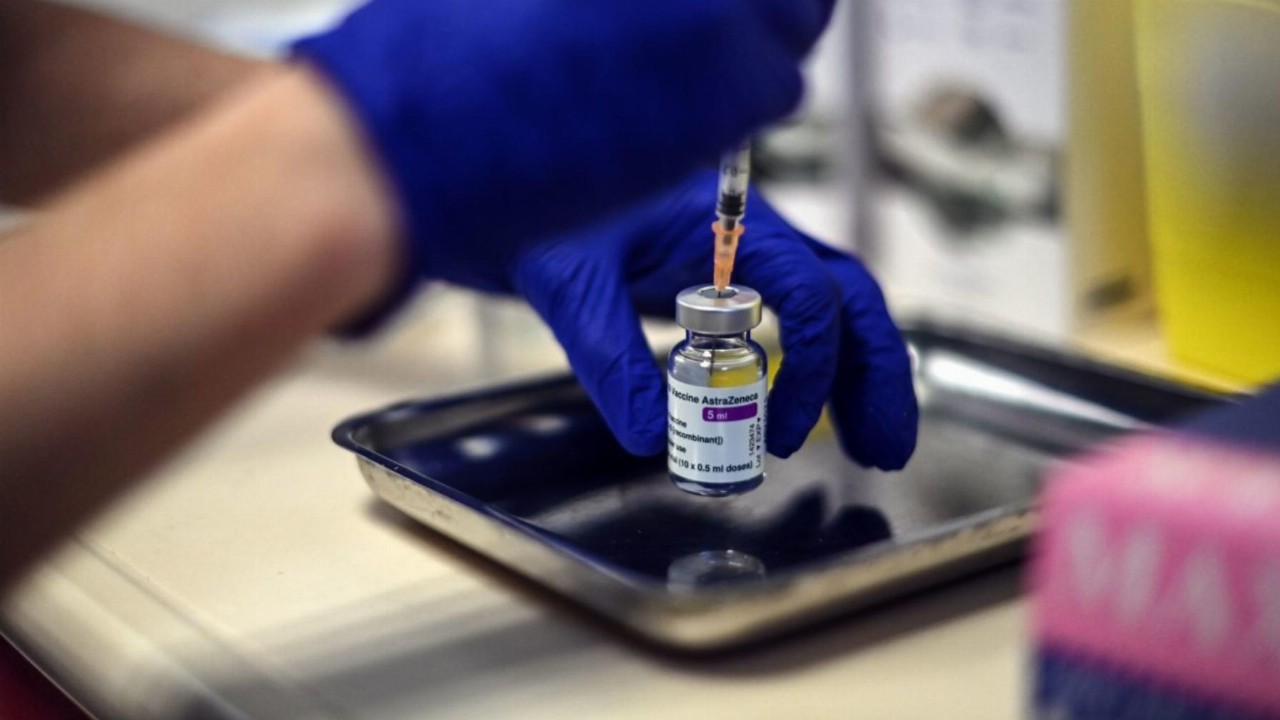
- The Therapeutic Goods Administration (TGA) has approved the AstraZeneca-Oxford University vaccine for use in Australia
- Those aged 18 and older are eligible for immunisation, which consists of two doses administered between four and 12 weeks apart
- Australia’s approval follows the vaccine’s emergency use listing by the World Health Organisation on Monday
- The emergency use listing process is designed to help poorer nations without access to their own regulatory resources quickly approve medicines for new diseases
- More than 330 million doses of the AstraZeneca shot will be distributed to poorer countries from the end of February
The Therapeutic Goods Administration (TGA) has approved the AstraZeneca-Oxford University vaccine for use in Australia.
This is the second candidate to receive the go-ahead after the Pfizer-BioNTech vaccine was approved last month.
Those aged 18 and older are eligible for immunisation, which consists of two doses administered between four and 12 weeks apart
However, despite patients over 65 years of age demonstrating a strong immune response, the regulators said “there were an insufficient number of participants infected by COVID-19 to conclusively determine the efficacy in this subgroup.”
“The decision to immunise an elderly patient should be decided on a case-by-case basis with consideration of age, co-morbidities and their environment taking into account the benefits of vaccination and potential risks,” the TGA added.
Australia’s approval of the shot follows its emergency use listing by the World Health Organisation (WHO) on Monday, further expanding access to the vaccine throughout the developing world.
“We now have all the pieces in place for the rapid distribution of vaccines. But we still need to scale up production,” said Tedros Adhanom Ghebreyesus, WHO Director-General.
“We continue to call for COVID-19 vaccine developers to submit their dossiers to WHO for review at the same time as they submit them to regulators in high-income countries.”
The emergency use listing process is designed to help poorer nations without access to their own regulatory resources quickly approve medicines for new diseases, which could otherwise lead to delays.
The approval comes just days after a panel provided interim recommendations on the vaccine, saying two doses roughly eight to 12 weeks apart should be given to all adults. The vaccine was also recommended for use in countries with the South African variant of COVID-19.
Overall, WHO’s review found that the AstraZeneca vaccine met the “must-have” criteria for safety, and that its efficacy benefits outweighed its risks. It’s also been hailed for its lower cost and easier distribution compared to other candidates, such as the Pfizer-BioNTech vaccine, which was listed for emergency use in December.
More than 330 million doses of the AstraZeneca shot will be distributed to poorer countries from the end of February.