- Nova Eye Medical (EYE) maps out a regulatory approval pathway for its retinal rejuvenation laser treatment
- Following feedback from the US FDA, the company has defined a clinical study plan and approval pathway for the 2RT retinal rejuvenation laser for intermediate, age-related macular degeneration (iAMD)
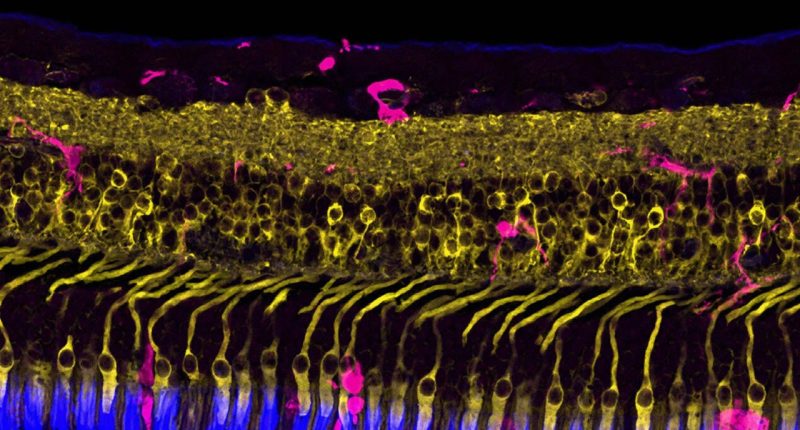
- 2RT is a world first, nanosecond laser therapy which stimulates the rejuvenation of cells in the retina to initiate a healing response, targeting the underlying causes of AMD
- Nova’s clinical study plan and commercial rights will be pursued by the company’s wholly owned subsidiary, AlphaRET
- Nova Eye Medical is up 12.5 per cent, trading at 27 cents
Nova Eye Medical (EYE) has mapped out a regulatory approval pathway for its retinal rejuvenation laser treatment.
Following feedback from the US Food and Drug Administration (FDA), the company has defined a clinical study plan and approval pathway for the 2RT retinal rejuvenation laser for intermediate, age-related macular degeneration (iAMD).
2RT is a proprietary, world first, nanosecond laser therapy to treat iAMD. The treatment stimulates the rejuvenation of cells in the retina to initiate a healing response that targets the underlying causes of AMD.
Treatment options for iAMD patients are currently limited. For patients with AMD in its late ‘dry’ form there is no treatment, while 10 per cent of patients with the ‘wet’ form require invasive ocular injections administered over many years, typically every four to eight weeks.
Nova said 2RT has the potential to transform the global management of AMD by treating patients earlier in the disease state. The treatment has been subject to a 20-year development program and has been shown in studies to reduce regression of iAMD by 77 per cent.
“As we pioneer this novel ground-breaking therapy using 2RT to transform the treatment of intermediate iAMD, we acknowledge that the dialogue with the FDA has been time consuming. It has also been complex,” Managing Director Tom Spurling said.
“We believe that the extent of the dialogue with the FDA demonstrates the potential importance of 2RT and it is a very positive achievement to have now established a clear path forward.”
The clinical study plan and the commercial rights will be pursued by the company’s wholly owned subsidiary, AlphaRET.
Partnering discussions are now underway for a clinical study.
Nova Eye Medical was up 12.5 per cent, trading at 27 cents at 12:43 pm AEST.