- Next Science (NXS) signs a multi-year distribution agreement for the sale and distribution of its antimicrobial wound gel, BlastX, in Australia and New Zealand
- The agreement was penned with Oraderm Pharmaceuticals for an initial term of five years, which will be automatically extended for another three years
- The new distribution agreement grants Oraderm exclusive rights across the two countries for the sale and marketing of a dual labelled version of BlastX
- Next Science is scheduled to deliver training to both the Oraderm and Douglas Pharmaceuticals’ sales team in June, with commercial sales in Australia and New Zealand scheduled for the second half of the year
- Next Science was up 8.54 per cent, trading at 89 cents per share at 2:15 pm AEST
Next Science (NXS) has signed a multi-year distribution agreement for the sale and distribution of its antimicrobial wound gel, BlastX, in Australia and New Zealand.
The agreement was penned with Oraderm Pharmaceuticals, a medical sales joint venture between Douglas Pharmaceuticals and Arrotex Pharmaceuticals, both of which are the largest privately-owned pharmaceuticals companies in New Zealand and Australia, respectively.
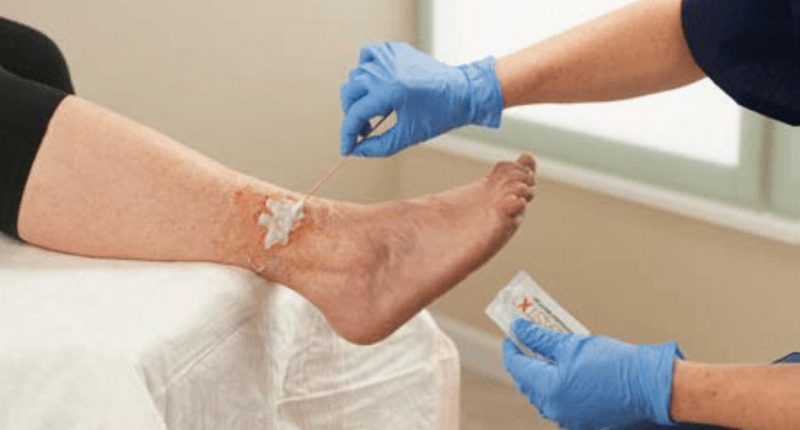
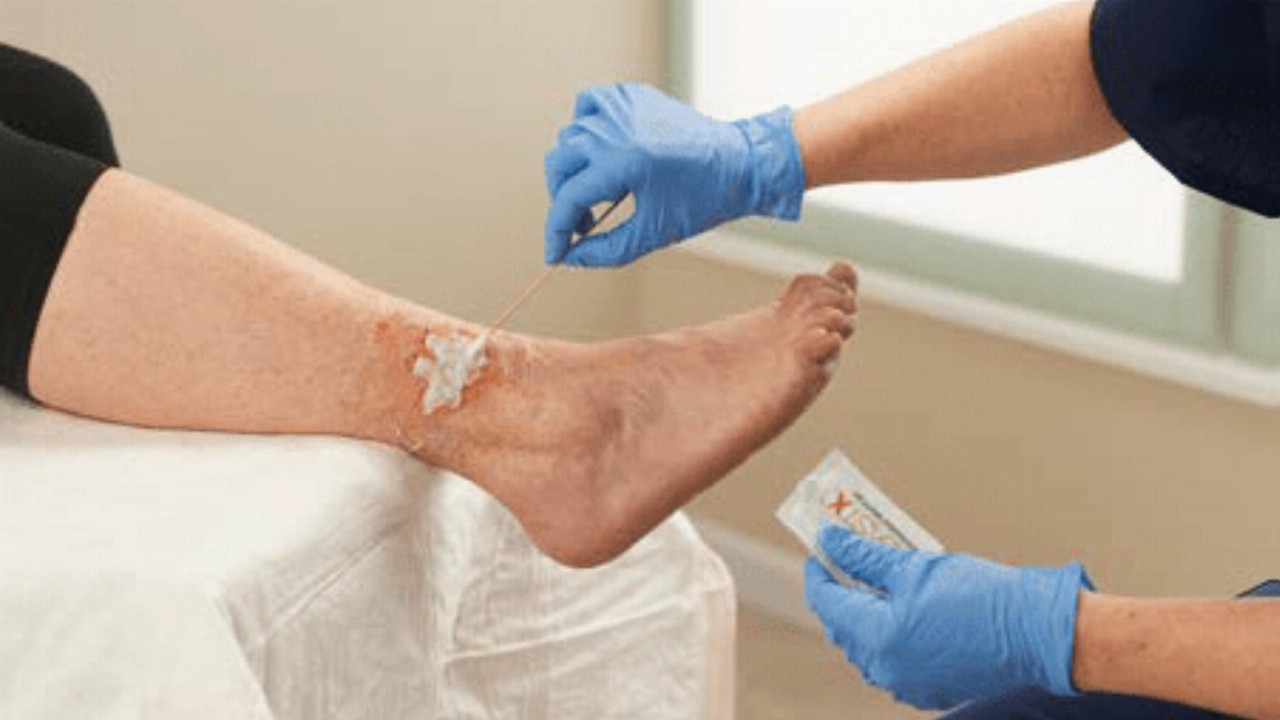
BlastX is a gel that works to deconstruct bacterial biofilm by enveloping and eliminating bacteria and then maintaining a moist wound environment conducive to healing. The product is considered ideal for the treatment of non-healing wounds, such as foot and leg ulcers and bed sores.
BlastX was cleared by the Australian Therapeutic Goods Administration (TGA) in May last year, allowing sales of the gel in Australia and New Zealand, along with FDA clearance and CE marking.
The new distribution agreement, spanning an initial five years, grants Oraderm exclusive rights across Australia and New Zealand for the sale and marketing of a dual labelled version of BlastX. The deal will automatically be renewed for another three-year period subject to each party’s termination rights.
Revenue from the agreement will be based on an agreed unit price and a minimum quantity per order.
Next Science Managing Director Judith Mitchell said the company is delighted to expand its commercial footprint for BlastX in Australia and New Zealand through Oraderm.
“This partnership enhances our ability to offer our proven wound care product to healthcare professionals and patients in Australia and New Zealand as we continue to pursue our mission to heal patients and save lives worldwide by reducing the impacts of biofilms on human health,” Judith Mitchell said.
Next Science is scheduled to deliver training to both the Oraderm and Douglas Pharmaceuticals’ sales team in June, with commercial sales in Australia and New Zealand scheduled for the second half of the year.
Oraderm said it will then turn its focus in Australia to hospitals, nursing homes, dermatologists and over-the-counter pharmacy sales.
Next Science was up 8.54 per cent, trading at 89 cents per share at 2:15 pm AEST.