- AFT Pharmaceuticals are travelling towards a clinical trial of its drug ‘Pascomer’ after settling a deal with dermatology drug developers Timber
- Pascomer is a skincare drug for treating Tuberous Sclerosis – a disease said to affect over 30,000 people in the U.S.
- Timber will be paying for clinical trials of Pascomer for a planned 120 patients in eight study centres across the U.S., Canada and Mexico
- AFT claims if the trials are successful, treating Tuberous Sclerosis patients could be an industry worth over US$300 million
Distributor and marketing company AFT Pharmaceuticals has secured a licensing and clinical deal for its skincare drug, Pascomer.
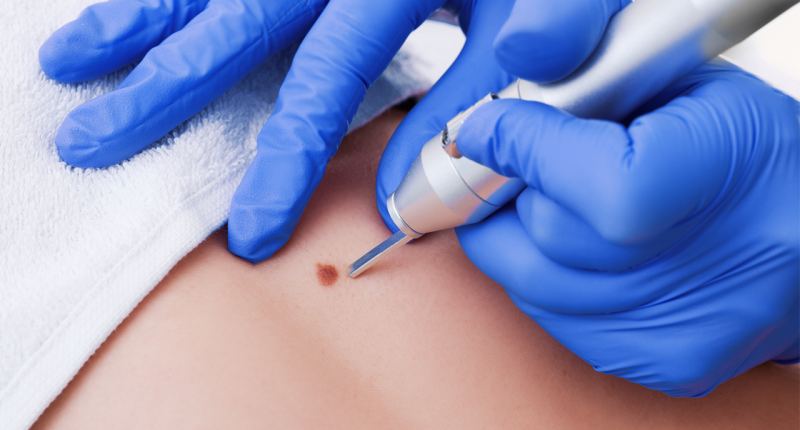
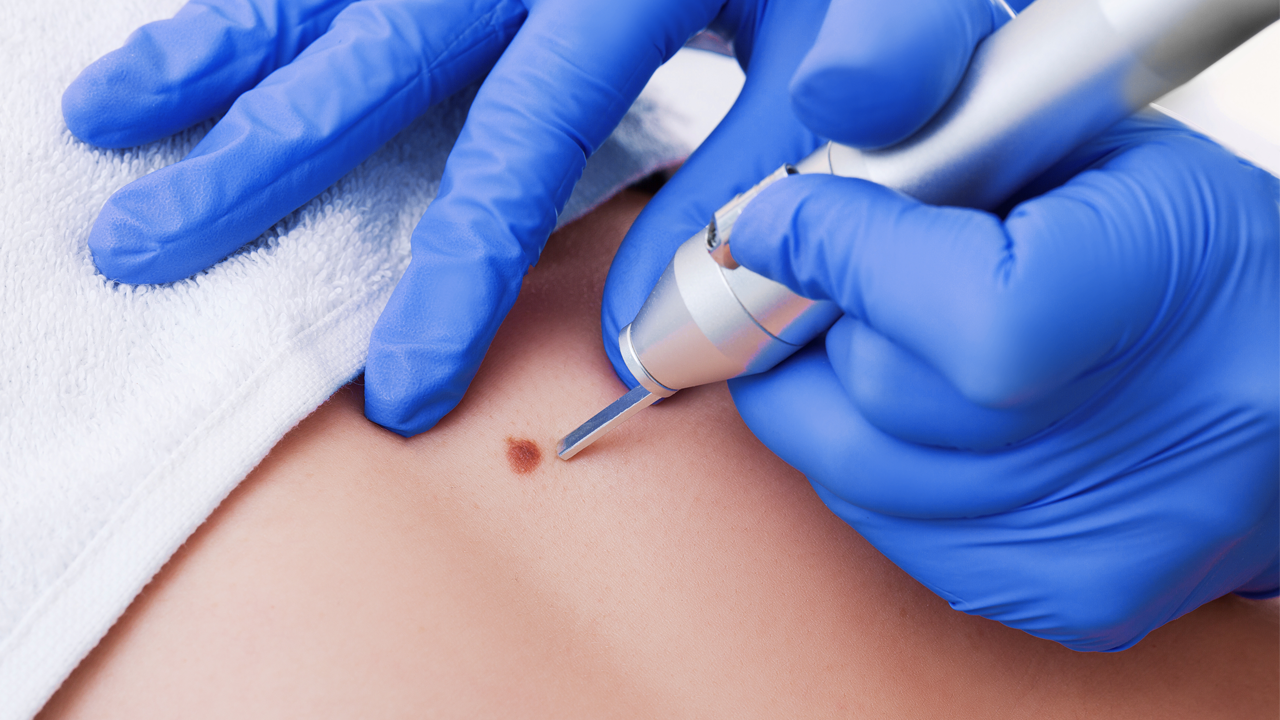
Inked with dermatology drug developers Timber, Pascomer will be reaching test patients in the U.S., Canada and Mexico. Pascomer is applied to the face of Tuberous Sclerosis patients – a disease said to affect over 30,000 people in the U.S.
AFT management claims that if clinical studies for Pascomer and its treatment are successful, helping Tuberous Sclerosis patients would be an industry worth over US$300 million.
Clinical studies for Pascomer are planned for 120 patients across eight study centres. Additional research centres in Australia, Spain, the U.K. and New Zealand will also take part. Full results are expected in 2020.
“The deal we have struck with Timber, mitigates AFT’s research and development risks, while still promising strong returns for the company if the clinical trials proceed successfully,” said AFT CEO Dr Hartley Atkinson.
AFT will receive milestone payments of US$10 million from Timber as clinical studies proceed successfully. However, AFT says its profit guidance for the 2020 financial year will remain unchanged, staying between NZ$9-12 million.
“Facial angiofibromas are a disfiguring condition affecting patients from childhood. So, a successful Pascomer development will offer an important therapeutic option to these sufferers,” said Hartley.
Pre-clinical development work has been completed for planning the upcoming trials – alongside approvals from the FDA. Timber will cover costs for the trial and payment of AFT’s staff.
“AFT’s dermal delivery technology coupled with Pascomer is potentially a significant breakthrough for people with facial angiofibromas,” added Timber President Zach Rome in this morning’s ASX announcement.
Shares in AFP are untouched today, stagnant at $2.91 a share. The company’s market cap is valued at $283.1 million.