- Medical Developments International (MVP) shares jump after the US Food and Drug Administration lifts its hold on pain relief drug, Penthrox
- This means that the company can immediately begin preparing for its phase three US clinical trial
- The two-year trial will be conducted on a targeted trauma and associated pain patient group and the company expects to start recruitment later this year
- MVP shares were up 27.6 per cent trading at $4.35
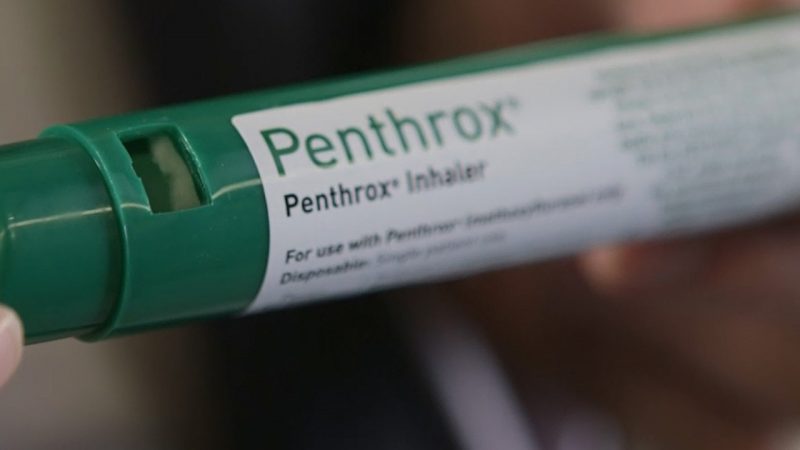
The US Food and Drug Administration (FDA) has lifted its hold on Medical Developments International’s (MVP) pain relief drug Penthrox.
This means that Victoria-based MVP can immediately begin preparing for its phase three US clinical trial.
Penthrox is a fast onset, non-opioid analgesic indicated for pain relief in patients.
The two-year trial will be conducted on a targeted trauma and associated pain patient group and MVP expects to commence recruitment later this year.
CEO Brent MacGregor said he was thrilled with the news, which allows the company to move forward with preparations for the trial.
“After years of hard work, our team is buoyed by the prospect of bringing the many benefits of Penthrox to the US market,” he said.
“This will strongly complement our growing Penthrox success in Europe, and fully supports our further investment in the product.”
Chair Gordon Naylor said the news is important because it brings Penthrox a step closer to licensing in the US market and because the FDA is seen as a global regulatory leader.
“Our renewed focus on the core business continues to yield results,” he said.
On the market, MVP was up 27.6 per cent and was trading at $4.35 per share at 12:45 pm AEDT.