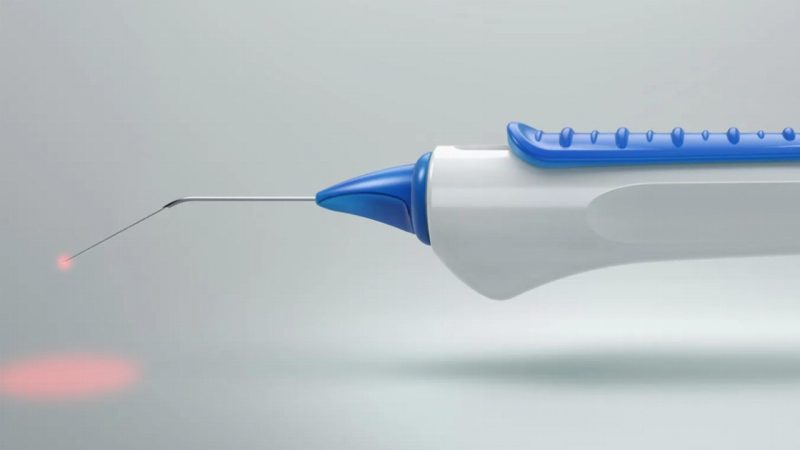
- Nova Eye Medical’s (EYE) iTrack Advance device has been cleared for sale to surgeons in the US to treat glaucoma
- The US Food and Drug Administration has cleared the newest generation of Nova’s canaloplasty device, paving the way for immediate marketing and sales
- The hand-held delivery system builds on EYE’s original device and is expected to appeal to a larger market, including cataract and comprehensive surgeons
- Nova has planned a full product launch at the American Society of Cataract and Refractive Surgery annual conference in San Diego in May 2023
- EYE shares are up 7.1 per cent to 30 cents at 12:54 pm AEDT
Nova Eye Medical’s (EYE) iTrack Advance device has been cleared for sale to surgeons in the US to treat glaucoma.
The US Food and Drug Administration cleared the newest generation of Nova’s canaloplasty device, paving the way for immediate marketing and sales.
The new hand-held delivery system builds on the company’s original iTrack device, used by glaucoma surgeons, and is expected to appeal to a larger market including cataract and comprehensive surgeons.
Clinical Professor at the Dean McGee Eye Institute of the University of Oklahoma Mahmoud Khaimi said the new design made the canaloplasty procedure efficient and “very ergonomic” for surgeons.
“We’ve taken the original iTrack device and incorporated it into an ergonomic handpiece that allows surgeons to easily advance and retract the microcatheter through the canal,” Dr Khaimi said.
“I am confident that surgeons will adopt this device into their treatment algorithm, either as an adjunct to cataract surgery or as a standalone procedure.”
With the FDA clearance, Nova has planned a full product launch at the American Society of Cataract and Refractive Surgery annual conference in San Diego scheduled for early May 2023.
EYE shares were up 7.1 per cent to 30 cents at 12:54 pm AEDT.