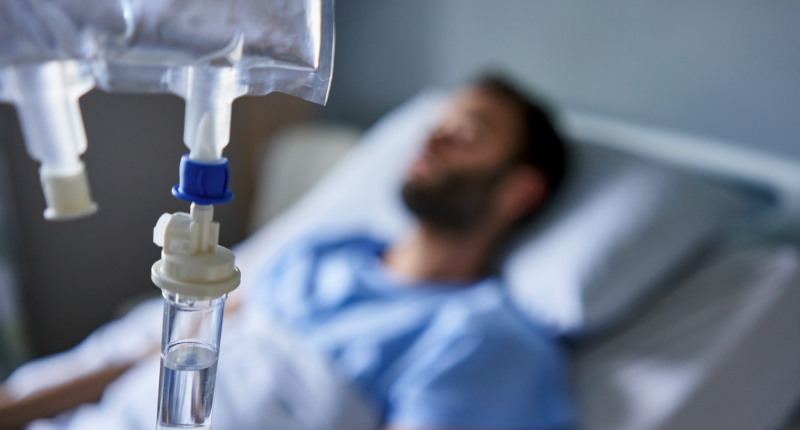
- Recce Pharmaceuticals (ASX: RCE) (FSE: R9Q) announces the successful dosing of the latest group of human participants with RECCE 327 (R327)
- Administered intravenously at a rate of 20 minutes and a dosage of 3,000mg
- RCE last traded at 44 cents, at 12pm AEDT
Recce Pharmaceuticals (ASX: RCE) has announced the successful dosing of the latest group of human participants with RECCE 327 (R327).
Administered intravenously at a rapid rate of 20 minutes and a dosage of 3,000mg, this marks a major achievement in the Phase I/II UTI/Urosepsis clinical trial.
The company is investigating various infusion durations at the 3,000mg dosage level.
This range is considered the optimal therapeutic window for R327’s dosing, and the company has confirmed it is safe for participants.
“We’re pleased to continue advancements within and surrounding our R327 clinical trials,” Recce Pharmaceuticals CEO James Graham said.
The additional infusion time at 3,000mg over 20 minutes highlights a compelling safety profile with the potential to treat the millions of patients worldwide that suffer from UTI/Urosepsis each year.”
In a separate clinical program, R327 has shown promising efficacy when applied topically against infections related to Diabetic Foot Ulcers, showcasing its potential to combat a diverse array of antibiotic-resistant infections.1
Further R327’s results will be disclosed upon the completion of the clinical trial, in line with study protocol.
RCE last traded at 44 cents, at 12pm AEDT.