- ResApp Health (RAP) has entered an agreement with Coviu to integrate its respiratory app into the company’s telehealth software platform
- The ResAppDx-EU app is designed to complete diagnostic tests to check for acute respiratory diseases
- Over 5500 clinicians in Australia use the telehealth platform
- At ResHealth’s AGM, the company tabled integration into Coviu as a key goal for the next six months
- ResApp Health gained 3.7 per cent this morning with shares trading for 28 cents apiece
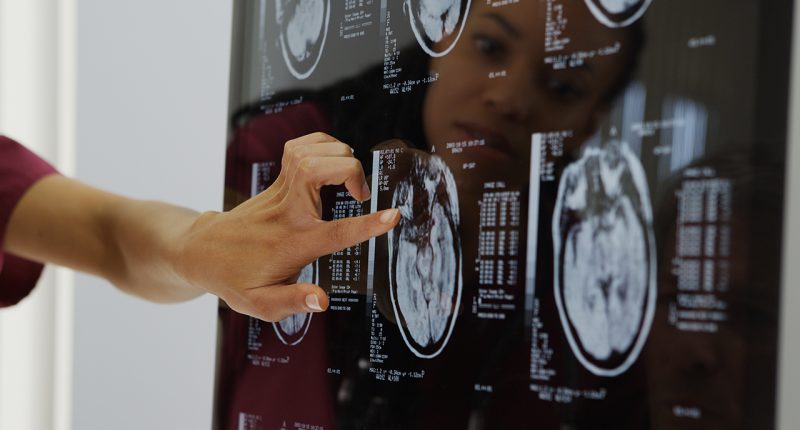
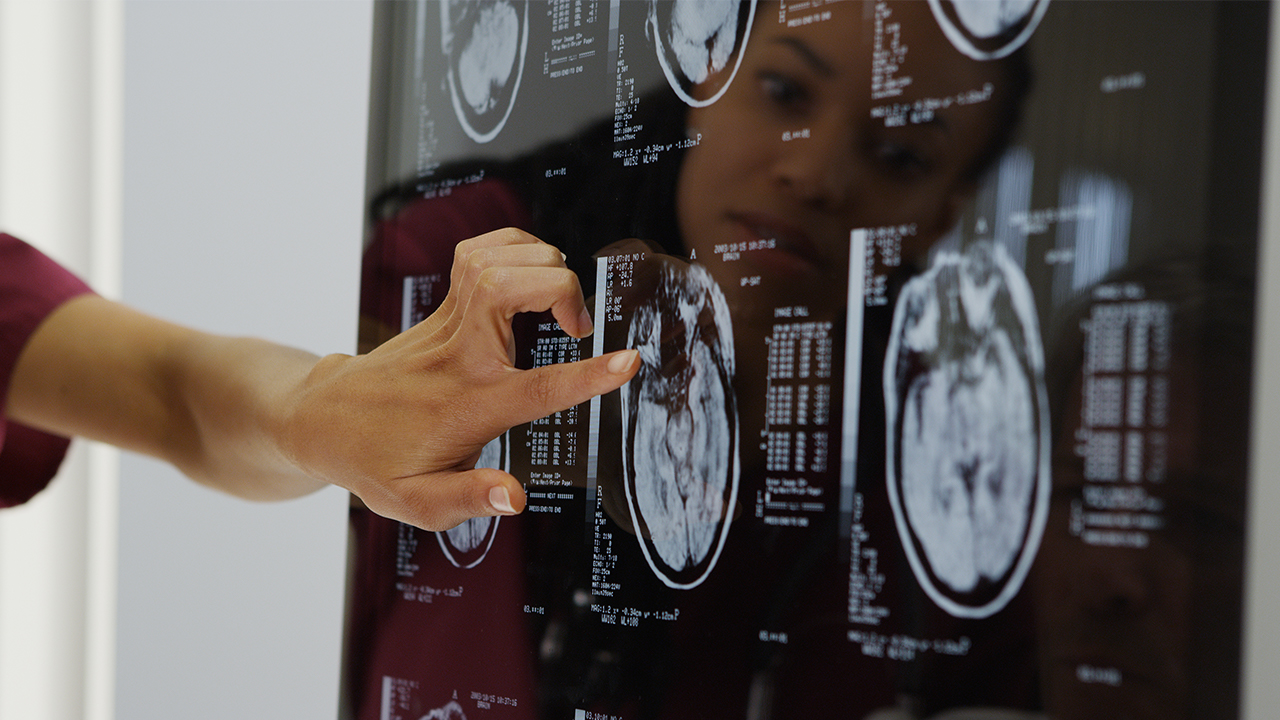
ResApp Health (RAP) has entered an agreement with Coviu to integrate its respiratory app into the company’s telehealth software platform.
The app, ResAppDx-E, is designed to complete diagnostic tests to check for acute respiratory diseases. The test uses machine learning algorithms to diagnose and measure the severity of respiratory conditions based on sound.
Under the joint development MoU, the two companies will work on integrating the ResAppDx-E respiratory diagnostic test into Coviu’s existing telehealth platform.
Work over the next four months will focus on designing a system to ensure the technology is used in appropriate circumstances and followed by the appropriate actions after testing is complete.
Additionally, both ResApp Health and Coviu will negotiate a cost model for the partnership before the end of the agreement.
The Coviu platform was first created by the CSIRO when researching telehealth delivery. Now, over 5500 clinicians across Australia use Coviu to provide consultations via video-call, in particular to people in regional and remote Australia.
Clinical studies in both Australia and the United States found ResAppDx-E accurately diagnoses lower respiratory tract disease, upper respiratory tract infections, asthma/reactive airway disease, pneumonia, bronchiolitis, croup, chronic obstructive pulmonary disease and obstructive sleep apnoea.
At ResHealth’s AGM today, the company tabled the integration of ResAppDx-E into Coviu as a key goal for the next six months.
Other milestones the company hopes to achieve in the next six months include a TGA decision on ResAppDx-EU for adults use in Australia and a FDA decision on paediatric use in the USA.
During the last financial year, the company received positive results from an at-home sleep apnoea study and plans to make a submission for CE Mark approval in Europe for its sleep apnoea screening product.
An agreement was also reached to join the Startup Creasphere program with pharmaceutical company Sanofi.
In addition, ResApp Health announced it would develop handheld and wearable devices. A prototype is slated for completion within six months.
ResApp Health gained 3.7 per cent this morning, and shares were trading for 28 cents each at 12:25 am.