- Singular Health (SHG) is given 510(k) clearance from the US Food and Drug Administration (FDA) for its 3Dicom MD software-as-a-medical-device product
- The company said the clearance represents a “significant step forward” in Singular Health’s commercialisation plan as it enables the commercial launch of its software into the US
- SHG plans to commercialise through a combination of enterprise partnerships and distribution agreements and through the company’s direct online sales channel
- Shares in Singular Health have ended the day up 19.1 per cent at 12.5 cents
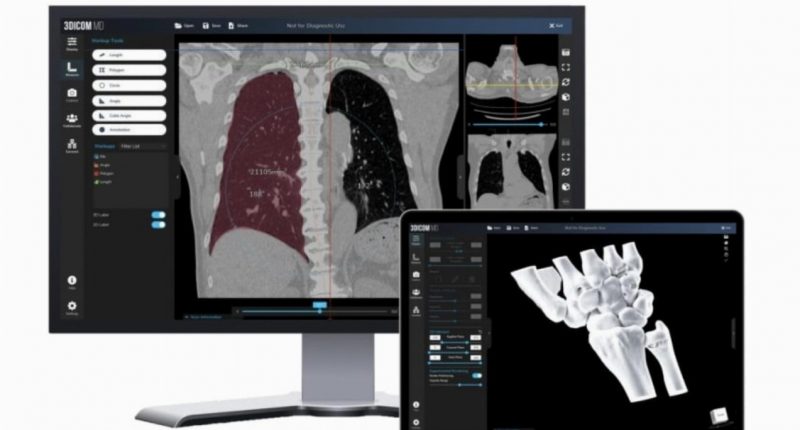
Singular Health (SHG) has been given 510(k) clearance from the US Food and Drug Administration (FDA) for its 3Dicom MD software-as-a-medical-device (SaMD) product.
The product is designed for medical practitioners to use and collaboratively visualise standard CT/MRI and PET scans in 2D and 3D in real-time with built-in voice and text chat, and remote control functionality.
The company said the clearance represents a significant step forward in commercially launching its software into the US, where the medical imaging software market was valued at US$885 million (A$1.4 billion) in 2020.
SHG plans to commercialise the product through a combination of enterprise partnerships and distribution agreements, as well as through the company’s direct online sales channel.
CEO and Managing Director Thomas Hanly reiterated the statement, adding that it provides a “regulatory moat and external validation of its software”.
“Furthermore, 3Dicom MDTM is a key element in the company’s Scan to Surgery strategy providing pre-surgical diagnostic review, planning and patient education.”
The company will now turn to focus its attention on commercialising the software in 2023.
Shares in Singular Health were up 19.1 per cent to close at 12.5 cents.