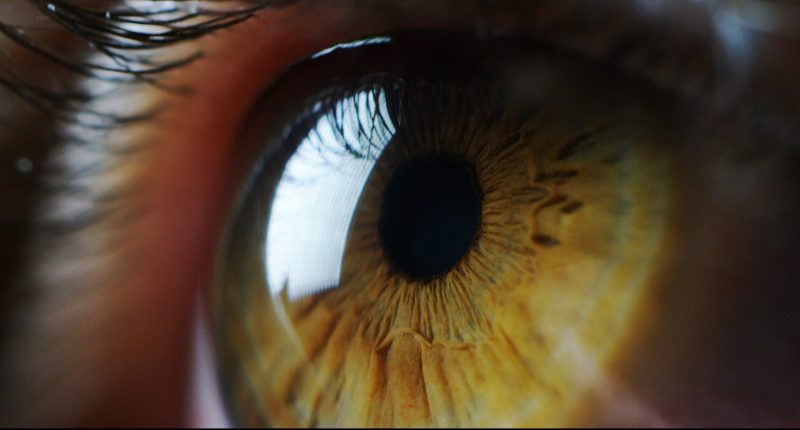
PYC Therapeutics (ASX:PYC) has reported its investigational drug candidate, VP-001, has shown success in patients with Retinitis Pigmentosa type 11 (RP11) in Phase 1/2 clinical trials where improvements were reported by patients for up to 18 months after treatment.
Listen to the HotCopper podcast for in-depth discussions and insights on all the biggest headlines from throughout the week. On Spotify, Apple, and more.
That was observed on the back of comparisons to RP11 patients who did not receive VP-001 as well as a “natural history control group.” VP-001 is reportedly showing improvements in patients conducting eye tests in dim lighting; as well as other technical measurements of sight.
The company reported on Friday that safety and tolerability measurements remain favourable with no serious adverse events in patients dosed to date.
What all of this underpins is the company’s upcoming approach toward the US FDA to negotiate on a registrational trial design ahead of the commencement of that study in 2026.
“These results help inform PYC’s final proposed design for the registrational trial of VP-001 in patients with RP11. The Company will meet with the US FDA in Q1 2026 to align on the data required to support a New Drug Application for VP-001 in RP11,” PYC Therapeutics wrote on Friday.
On Friday, with every sector in the red and a good mood hard to find, the stock has sunk over -6%, to the dismay of some shareholders in the HotCopper forums.
PYC last traded at $1.24/sh.
Join the discussion: See what HotCopper users are saying about PYC and be part of the conversations that move the markets.




-1200x645-380x200.jpg)


