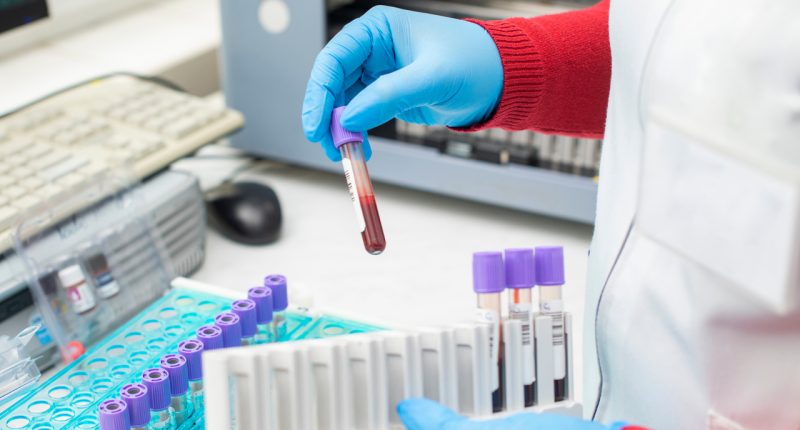
- Resonance Health has filed a provisional patent for the discovery and use of novel blood markers to determine iron status
- These blood markers have been identified as part of the company’s molecular medicine research and development iron program
- The current test is routinely used however it can be affected by the presence of many other conditions such as obesity, inflammation and infections
- Resonance is currently up 7.41 per cent with shares trading for 14.5 cents apiece
Resonance Health has filed a provisional patent for the discovery and use of novel blood markers to determine the iron status.
These blood markers have been identified as part of Resonance’s molecular medicine research and development program.
Presently a very common blood test, serum ferritin (SF), is used as a proxy for the iron status of a patient.
A ferritin test measures the amount of ferritin (protein) in the blood and it helps doctors understand how much iron is being stored in the body.
Low ferritin results indicate that the body’s iron stores are low and have an iron deficiency, while higher levels indicate that the body has a condition which causes it to store too much iron.
It could point to liver disease, rheumatoid arthritis and other inflammatory conditions. Some types of cancers may also cause blood ferritin to be higher than normal.
Globally, clinicians use SF as a diagnostic tool and as a substitute for MRI to monitor liver iron in thalassemia (blood disorder involving lower-than-normal amounts of oxygen-carrying protein) and other iron loading conditions where access to MRI is limited or expensive.
Although routinely used, SF is an acute phase reactant and can be affected by the presence of many other conditions such as inflammation, infections, obesity and cancer.
Once SF reaches a saturation point, the connection between SF and total body iron stores breaks down.
Consequently, as the liver iron concentration (LIC) reaches this saturation point, it becomes more challenging for a clinician to assess risk, determine chelator dosing and effectively monitor response to therapy.
While the use of FerriScan (R2-MRI) remains the global standard for measuring LIC, Resonance is actively pursuing alternative biochemical methods to assist clinicians to diagnose and monitor iron overload in locations where MRI is limited.
In a recent study of 59 patients, 30 thalassemics and 29 normal, who underwent MRI for LIC quantification, Resonance reports that a combination of three newly identified biomarkers performed better than SF in predicting LIC.
Due to this result, Resonance has now filed a provisional patent for the discovery of these biomarkers and will continue to investigate their usefulness as an alternative screening and monitoring tools in patients with suspected iron overload.
However, specific details regarding the novel blood markers will remain confidential awaiting the patent and due to additional data gathering and analysis.
Resonance is currently up 7.41 per cent with shares trading for 14.5 cents apiece at 3:24 pm AEDT.