- Uscom has received the Conformité Européenne (CE) certificate for three of its SpiroSonic digital ultrasonic spirometry devices
- SpriSonic is a reliable and digital pulmonary (type of high blood pressure) function testing device
- The company is looking forward to expanding internationally in the next 12 months
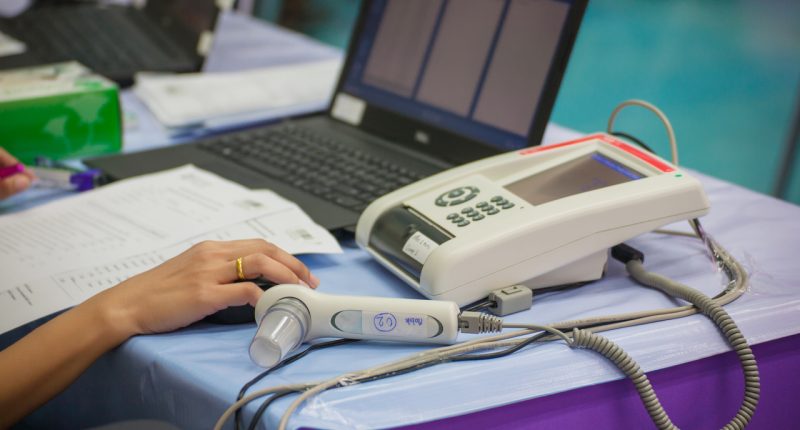
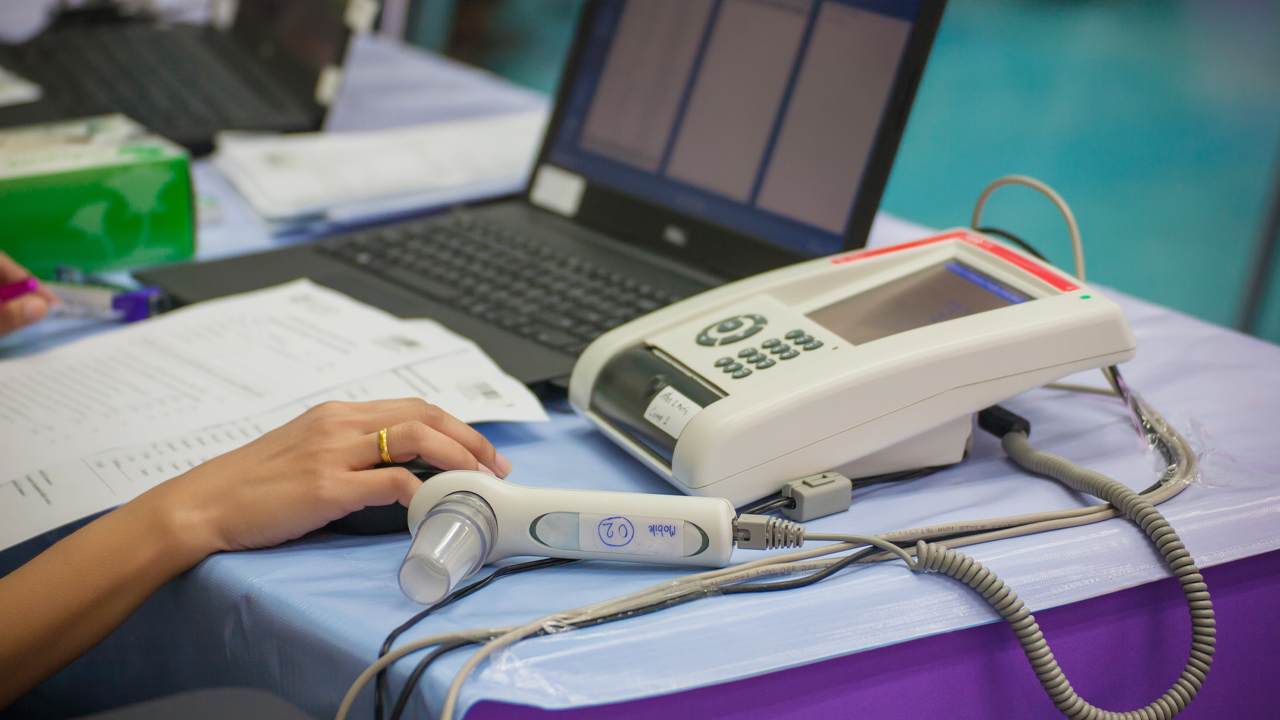
Uscom has received the Conformité Européenne (CE) certificate for three of its SpiroSonic digital ultrasonic spirometry devices.
The approval is for Uscom’s FLO, SMART and MOBILE devices.
A CE certificate is required to sell products within the European Economic Area (EEA) and is a declaration by the manufacturer that the product meets the European Directives.
SpriSonic is a reliable and digital pulmonary (type of high blood pressure) function testing device which uses ultrasound technology. It can be used in research, clinical and home care environments.
The device is similar to an alcohol breath test, it assesses the lung function by measuring how much (volume) and at what speed (flow) air can be inhaled and exhaled.
The test helps to provide information on the patient’s current lung function, disease progression and the effect of medication.
Sprisonic help assess chronic obstructive pulmonary disease (COPD), sleep disordered breathing, asthma and industrial lunch disease. It can also be connected to mobile phones through its app.
CE re-approval was required due to the relocation of Uscom Kft to Budapest. The relocation was needed to expand the facility as an increase in sales is expected once the Chinese NMPA certification is received.
Chairman Rob Phillips is pleased with the approval and says that it has a lot of partners keen to advance sales.
“The CE certification of our digital ultrasonic spirometers allows us to sell into the European, and most SE Asian and Middle Eastern markets,” Rob said.
Uscom is a medical technology company that is specialising in development and marketing of non-invasive cardiovascular and pulmonary devices. It has three leading devices: USCOM 1A, Uscom BP+ and Uscom Spirosonic.
USCOM 1A is a haemodynamic (heart and blood) monitor that measures cardiovascular function, detects irregularities and is a used to guide treatment.
The Uscom BP+ is a central blood pressure monitor that measures blood pressure and blood pressure waveforms in the heart and arms.
The company is looking forward to expanding internationally in the next 12 months.
“We are expecting the flow of new approvals from Europe and China to continue over the next 12 months, including approval for our new and exciting SpiroSonic AIR device. We anticipate this device will significantly impact the digital home care asthma and COPD market once approved,” he said.
Uscom’s new product SpiroSonic AIR device is still undergoing CE certification.