- EMVision Medical Devices (ASX:EMV) assembles an advanced 28-antenna prototype for its second-generation helmet scanner
- The ultra-lightweight helmet scanner is designed for use in road and air ambulance settings
- An ethics submission for healthy human volunteer testing has been filed and is anticipated to commence in the coming months
- The second-gen is on track to be assembled in the first half of 2024 and will be used for road/air ambulance trials under EMVision’s collaboration with the Australian Stroke Alliance
- EMV shares were up 6.46 per cent, trading at $1.57 at 1:09 pm AEDT
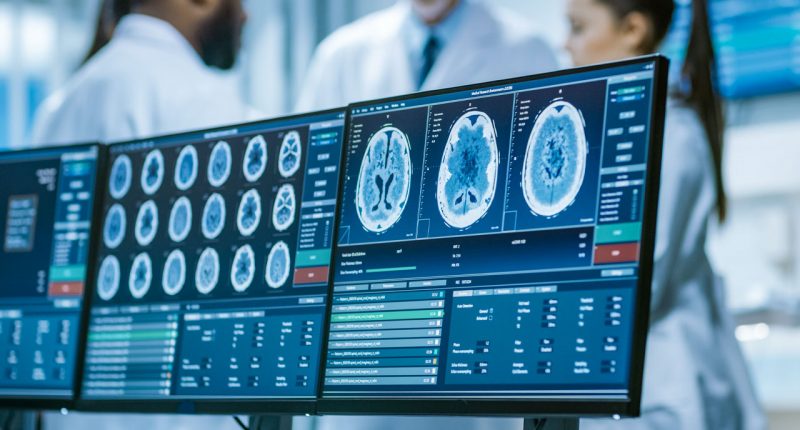
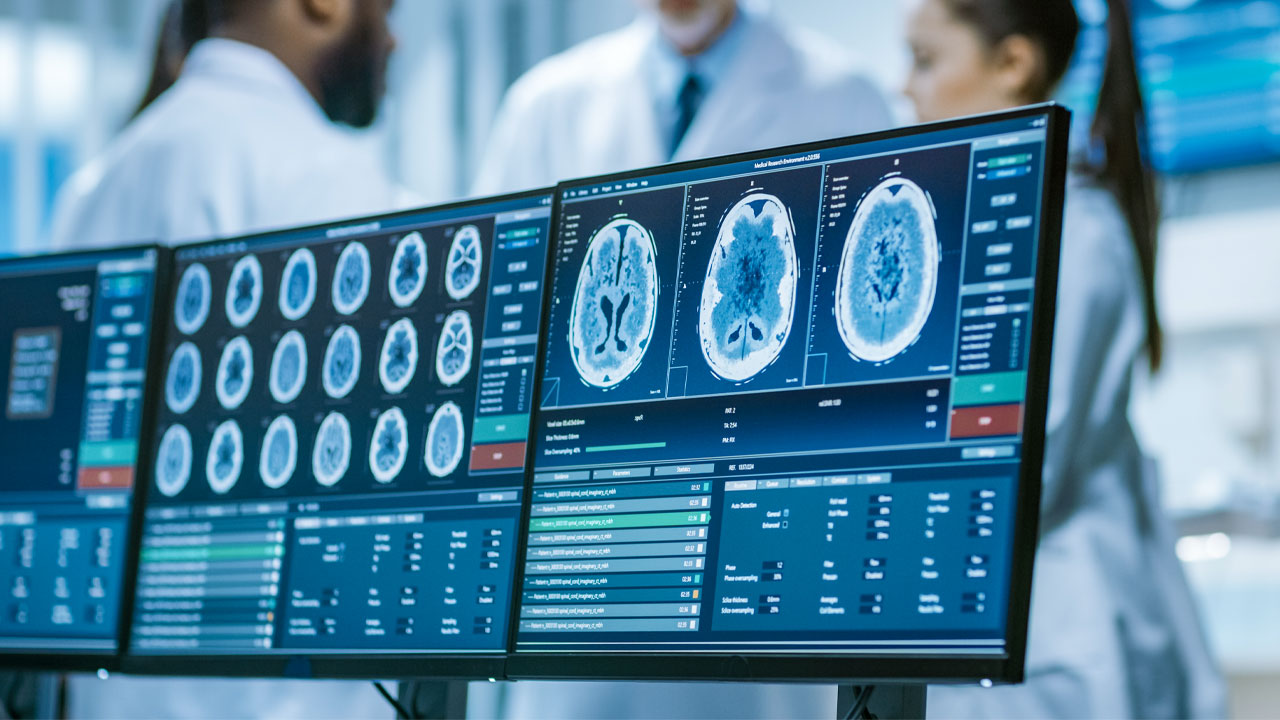
EMVision Medical Devices (ASX:EMV) has assembled an advanced 28-antenna prototype of its second-generation helmet scanner.
The ultra-lightweight product is designed for use in road and air ambulance settings.
Product features
The product complements EMVision’s existing product range, addressing the substantial clinical demand in both bedside brain imaging (first generation, currently undergoing multi-site clinical trials) and initial responder brain imaging (second generation).
Weighing under 10 kilograms, it’s purpose-built for easy transportation to point-of-care locations through a convenient backpack.
It incorporates an advanced 28-antenna 3D array, providing complete coverage of the entire brain in a single scan, all while maintaining high performance with lightweight antennas.
The second generation is engineered to be both reusable and replaceable, while the coupling media will be a consumable item required for each scan.
The core algorithms initially developed for the first generation are set to be adapted and upgraded for the second-generation device.
Bench testing process
The bench testing process will assess various technical parameters in comparison to simulations, leading to target detection investigations.
An ethics submission for healthy human volunteer testing has been filed and is anticipated to commence in the coming months.
In relation to regulatory strategy for the second generation, EMVision plans to leverage the first generation as a predicate device to follow the FDA 510(k) pathway. The pathway mandates that the device seeking clearance must demonstrate “substantial equivalence” to a predicate device that has already been considered safe and effective.
Proof of concept
This advanced prototype serves as a predecessor to the “proof of concept” system, designed for pre-hospital deployment.
This innovation represents a vital step forward for the Australian medical device company, offering a novel approach to non-invasive internal body imaging.
The second generation is on track to be assembled in the first half of calendar year 2024, It will then be used for planned road/air ambulance trials under EMVision’s collaboration with the Australian Stroke Alliance.
EMV shares were up 6.46 per cent, trading at $1.57 at 1:09 pm AEDT.